Research
The spinal cord traumatic or ischemic injury can lead to the loss of motor function and development of paraparesis or fully developed paraplegia often combined with muscle spasticity.
The important distinction between spinal cord ischemia and spinal cord trauma (which may occur in a diving or car accident for example), is that in spinal cord ischemia no mechanical damage has occurred to the spinal cord. Connectivity between the spinal cord and brain motor centers is present, but there has been a selective loss of small inhibitory neurons in the spinal cord that are necessary for coordinated motor activity. Loss of these neurons can lead to irreversible muscle spasticity and rigidity, or loss of muscle control, in the lower limbs.
After the spinal traumatic injury, the motor and sensory functions are lost because the descending motor fibers which originate in the brain and are responsible for the initiation of motor activity are physically injured or fully transected.
In general, the ability to control the limbs after a spinal cord ischemic or traumatic injury depends on two factors: the segmental level of the injury and the severity of injury to the spinal gray and white matter.
1) Transient spinal ischemia-induced spastic paraplegia is a devastating complication associated with aortic cross-clamp due to the reduced blood flow to the spinal cord. Depending on the duration of ischemic episode resulting neurological deficit can be presented as spastic or flaccid paraplegia. In ischemic spasticity, a population of small inhibitory interneurons is selectively lost (see figure below; panel “A”) leading to exacerbated A-motoneuronal activity and resulting muscle spasticity.
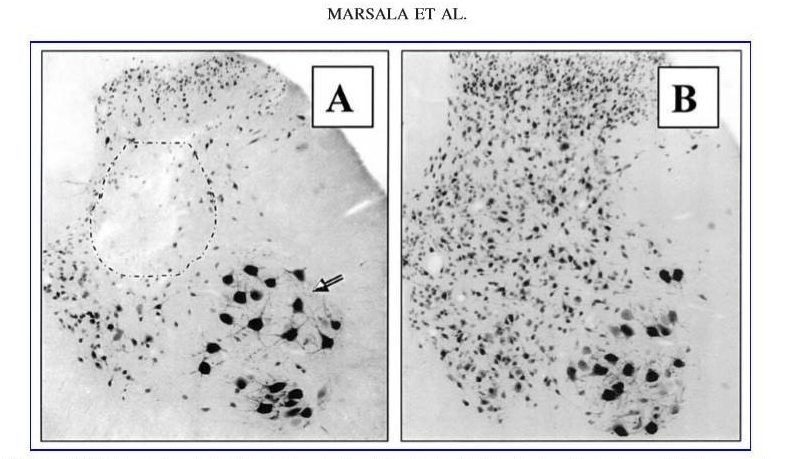
FIG. 6. (A) Histological analysis of the lumbar spinal cord in animal with fully developed ischemic spasticity shows a selective loss of small inhibitory neurons (black circle), but with continuing presence of ventral a-motoneurons (arrow). (B) Control.
2) Chronic spinal trauma-induced motor dysfunction is primarily the result of a mechanical injury into a descending motor tract and partial destruction of segmental interneurons and A-motoneurons at the injury site. Extensive multi-segmental spinal cord injury may lead to permanent paraplegia and is often associated with the development of spinal cord cavitation (ie syringomyelia; see inserted figure below).

Macroscopic images taken from transversely-sliced spinal cord (thickness of each slice is between 3 and 4 mm) at the injury site in the 2.5-kg compression group with 9 months survival. Extensive bilateral cavities can be identified. Positive integers indicate a location caudal to the injury epicenter (0). (B) 2D FSPGR magnetic resonance imaging (MRI) image of the same spinal cord as that seen in A–A′″ confirms the presence of bilateral septic cavitation, and areas of high-density structures around the injury epicenter (red arrow). (C) 3D rendered image of the same spinal cord shown in B. (D) 3D rendered image of another spinal cord in the 2.5-kg compression group showing disseminated smaller cavitations (scale bar in B=8 mm).
Our research in the field of spinal ischemic and traumatic injury aims at:
- development of cell-replacement therapies aimed at re-populating pools of inhibitory neurons lost after spinal cord ischemia
- characterize the behavioral-treatment effect of spinally-grafted cells on the recovery of motor function after spinal cord traumatic injury
- identify the optimal time frame for cell grafting after spinal ischemic or traumatic injury and the optimal dosing of grafted cells associated with most pronounced treatment effect
- improve spinal gene and cell delivery techniques in large animal models (pigs and non-human primates), that will identify the optimal route of gene or cells delivery and which can potentially be used in perspective human clinical trials in patients with spinal ischemic or traumatic injury